Treatment of skin carcinomas of the face by high-dose-rate brachytherapy and custom-made surface molds
Guix. IJROBP 2000;47:95
A total of 136 patients with basal or squamous cell carcinomas of the face were treated between March 1992 and March 1997 by surface molds and HDR brachytherapy with iridium-192. Nineteen patients were treated with standard Brock applicators and 117 patients with custom-made polymethyl methacrylate applicators, built over a plaster mold obtained of the patientís face. Minimum dose administered to the tumor was 6000 to 6500 cGy in 33 to 36 fractions at 180 cGy/fraction in lesions of up to 4 cm. Lesions greater than 4 cm were boosted up to 7500Ė8000 cGy after a 3-week pause.
Results: With the custom-made surface molds, the dose distribution was uniform in the surface of the skin and at 5 mm depth in the whole area of the applicator. Differences between the areas of maximum and minimum dose at this depth never reached values higher than 5% of the prescribed dose. At the edges of the custom-made molds dose gradient was sharp, with the detected dose at 5 mm from the applicator being negligible. All the patients were complete responders. There were 3 local recurrences, 1/73 patients treated for primary tumor and 2/63 patients treated for recurrent tumor. Actuarial local control at 5 years for all patients was 98%, for those patients with primary tumors 99%, and for recurrent patients 87%. The treatment tolerance was excellent in all cases. No severe, early, or late, complications were detected.
Conclusions: Radiotherapy is a highly effective treatment of skin carcinomas of the face. Custom-made molds, to be used in conjunction with HDR brachytherapy equipment, make possible a uniform dose distribution, with a sharp dose gradient in the limits of applicators. Custom-made surface molds are easy and safe to use, and they fit very accurately for daily treatment. Local control is excellent with minimal sequelae or complications. Probably they will become the standard way of treatment of face skin carcinomas in the near future.
|
|
 |
|
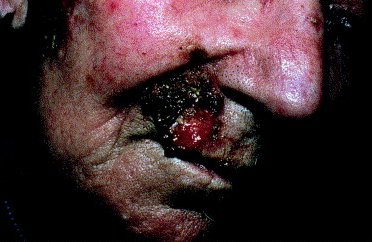
Before treatment. Squamous cell carcinoma recurred after surgery with perineural invasion. |
Result at 5 years after treatment with 6500 cGy, 3 weeks rest, and 1500 cGy boost. No recurrence; no late effects; complete remission was obtained with excellent cosmesis. |
Radiotherapy of skin carcinomas has been done, generally, with low-energy X-rays (90 kV or less) or electrons of a linear accelerator. Some cases have been treated with interstitial brachytherapy with 192Ir sources placed across the tumor volume, or wax molds with gamma ray emitters (Ra, Co, Au, Rn, Cs). The continuous technological advances produced in the field of brachytherapy have made possible the use of highly accurate after-loading equipment, with radioactive sources, usually 192Ir, of few millimeters in length and less than 1 mm diameter and high activity. This high-dose-rate (HDR) equipment is accompanied by programs of treatment planing and calculation of dose distribution incorporating sophisticated treatment optimization algorithms, which allow one to modify the dosimetry in order to obtain an individually adjusted dose distribution in each case
In the present report, we present a prospective series of patients with skin carcinomas of the face, treated in our institution by HDR brachytherapy, analyzing the treatment parameters and the results with respect to local control, cosmesis, and complications.
The treatment of 136 patients was performed with skin applicators connected to HDR equipment (Microselectron, Nucletron Int. BV). Two types of applicators were used: Brock type (Nucletron Int. BV) and custom-made molds The first type of applicators was employed in 19 cases, to treat tumors of up to 2 cm diameter localized in flat areas of the skin, that remained in the same horizontal plane with the patient in the treatment position, in order to assure its immobility during treatment. These applicators were available in 3 sizes: 1, 2, and 3 cm diameter. The radioactive source occupied only 1 active position in the center of the applicator at 15 mm from the surface of the skin. The treated area comprised the tumor volume plus a safety margin 5 to 10 mm of normal tissue.
The second type of applicator was used in the 117 remaining cases. It consisted of a mold of polymethyl methacrylate (PMMA) of 5 mm thickness, built over a plaster mold obtained as an individual impression of the region of the face to be treated. The construction of this PMMA mold was very similar to the construction of dental prostheses. First, an impression of the region of the patient to be treated was obtained with condensation silicones of putty texture (Optosil, Bayer), carefully adapted to the surface of the skin with gentle pressure. Over this impression a plaster model was obtained, with the same surface characteristics as the patientís face. Over this plaster model, the contours of the tumor were carefully drawn, requiring generally the presence of the patient. Over this plaster model, successive thin layers of acrylic material with catalyzer were deposited, until a minimum thickness of 5 mm was obtained, taking care to avoid sharp surfaces. This first layer of PMMA had to act as a bolus material and as a first support for the brachytherapy catheters. On it, the appropriate number of plastic tubes, covering the area to be treated, were fixed with an instant contact glue. Usually 3 to 7 parallel and equidistant tubes were placed, following the contour of the zone to treat, parallel to the skinís surface and avoiding sharp turns. The distance between the catheters ranged between 5 to 10 mm.
The custom-made applicators were used to treat tumors of more than 2 cm diameter, or those of smaller size but seated in a non-flat region or a difficult-to-fix area with the Brockís applicators. In one patient it was necessary to built a second mold at the halfway point of the treatment, due to changes in the surface of the skin resulting from tumor regression.
In all cases, the minimum tumor dose was of 6000 to 6500 cGy in 33 to 36 fractions of 180 cGy, calculated at 5-mm depth from the skin surface in the center of the Brockís applicator or with geometrical dose optimization in the custom-made molds. Patients with lesions 4 cm or more had a boost of 1800 cGy in 10 fractions, after a rest period of 3 weeks, with total dose ranging from 7500 to 8000 cGy.
Dose distribution of custom-made molds
In the custom-made molds it was possible to obtain a uniform dose distribution in the surface of the applicator as well as at 5 mm of depth from the skin surface in the whole area of the applicator. The difference between the maximum and minimal dose at those depths did not surpass the 5% of the prescribed dose. In the edges of the applicator, the dose gradient was sharp enough to include an almost negligible amount of normal tissue, as can be seen in the autoradiograph. The maximum radiation doses were observed in areas close to the catheters, whereas the minimum radiation dose was in the spaces between the plastic tubes. The TLD readings coincided with the calculated dose at the same points within a range of 5%, values that are between the margins of error for this type of dosimeter.
Tolerance of custom-made molds
All patients tolerated treatment without difficulties. Patients helped the nurses to fit the custom-made mold in place. Treatment time took 3 to 8 minutes in each session. The custom-made molds were very ease to use, and the patients felt comfortable during treatment. There were no cases of interruption of treatment resulting from break of the applicator or constriction of the plastic tubes preventing the radioactive source from traveling properly to the treatment dwell positions.
Local tumor control
All the patients had complete remission of their tumor. One hundred thirty-three patients of this study were free from recurrence at the closure time. A total of 3 patients had local recurrences. No patient had regional or distant metastases. Patients at risk for primary and recurrent tumors were, respectively, at 1 year 72 and 64; at 2 years 64 and 55; at 3 years 52 and 45; at 4 years 44 and 38; at 5 years 32 and 29; at 6 years 21 and 18; and at 7 years 12 and 11. The actuarial disease-free survival at 5 years by the Kaplan-Meier method was 98%. If we consider only the 73 patients treated at first intention for primary tumors, the local control at 5 years was 99%, whereas in the 63 patients treated for recurrences the 5-year control rate was 87%. In 111 (82%) cases, the macroscopic tumor complete remission was observed before the end of treatment, in 22 patients (16%) during the 4 weeks after ending treatment, in 2 cases (2%) between 4 and 8 weeks, and in 1 patient (1%) during the tenth week after treatment.
The local relapses occurred in 1 squamous cell carcinoma of the frontal region, 4 cm in diameter, at 23 months after treatment; in 1 recurrent basal cell carcinoma of the cheek, 5 cm long, 17 months after treatment; and in 1 second recurrence of a basal cell carcinoma of the nasal columnella, 2 cm in diameter, 23 months after completion of brachytherapy. Due to the few events recorded, no statistical analysis for risk factors of local recurrence could be performed.
Complications and cosmesis
All patients presented a certain degree of skin erythema at treatment completion, degree 1 in 117 cases and degree 2 in 19 cases. Fourteen patients (10%) had an ulceration degree 1 at 6 weeks after the end of treatment. This ulceration was dose-independent and was related to the size of the tumor and to whether the tumor was excised. In those patients with lesions completely excised at time of surgery (6 cases), skin ulceration only appeared for mold diameters larger than 2.5 cm and when 60 Gy were reached (2 cases). They resolved in 3 weeks. In those patients without excision of their lesion (130 cases), treatment was begun with skin ulceration due to the tumor. At the end of the first part of the treatment (65 Gy), ulceration was still present in all cases. It healed during the next 3 weeks in 73 patients. No biopsies were taken to determine if ulceration was due to tumor persistence or radiodermitis. The boost was given to 31 patients with lesions initially of 4 cm in diameter or more. Twenty-seven of them began treatment with ulceration still present. All 31 patients who were boosted presented ulceration at the end of treatment. At 3 weeks after completion of boost, 17 patients still had ulceration. At the 6 weeks after the treatment visit, there were 14 patients with skin ulceration present, 2 in the group of no boosted patients and 12 boosted patients. At 3 months after completion of treatment there were still 2 patients with skin ulceration, 1 boosted and 1 no boosted. At the fourth-month visit all patients were healed.
The relationship between the immediate tolerance to treatment and probability of local control, size of the lesion, and whether it was a primary or recurrent tumor was analyzed. No statistical differences were found except that recurrent tumors had worst tolerance to treatment than primary tumors.
The cosmesis and long-term complications were evaluated according to the presence of edema, alopecia, hypopigmentation or hyperpigmentation, telangiectasias, fibrosis, scars, skin atrophy, and skin retraction. A strong relationship was found between the time after completion of the treatment and the cosmesis. At 2 weeks after treatment, 38% of the patients had good or excellent cosmesis; at 3 months after the end of treatment, 92% of the patients had good or excellent cosmesis and 8% a poor cosmesis; and at 6 months from the treatment, cosmesis was considered to be excellent (no treatment sequelae) or good (minimal treatment sequelae) in 98% of the cases, with only 2% of the patients having unfavorable cosmesis. The unfavorable cosmesis at long term was observed in patients with recurrent tumors greater than 4 cm diameter that had destroyed wide areas of normal tissue. There were no cases of modification of the cosmesis between observations done later than 6 months after the end of treatment. There were no severe late complications related to treatment.
Discussion
Following the discovery of radium, wax molds with radium or radon seeds were used with excellent results in the treatment of skin carcinomas. Their use posed a huge radioprotection problem because they had to be manually loaded. With the introduction of megavoltage units and electrons beams, wax or paraffin molds fell into disuse. The last important report about this technique was from Ashby et al. in 1989 . They treated 642 patients with skin cancer other than melanomas, using wax and paraffin molds with radon sources. They achieved a local control of 96.8% with a long-term complication rate of less than 1%.
Lovett and colleagues published in 1990 the results obtained in a series of 339 patients with skin carcinomas treated by external radiotherapy. There were 189 treated with superficial X-rays, 57 with electrons, 15 with megavoltage photons, and 78 with a combination of beams. Given the number of patients included in this study and the follow-up period (20 years), it can be considered as representative of the results obtained with external beam radiotherapy. Tumor local control was strongly related to the kind of treatment used. Tumor local control was 95% (180/189) for those patients treated with superficial X-rays, 77% (44/57) for those treated with electrons, 66% (10/15) for megavoltage photons, and 76% (60/78) for those treated by mixed beams. The overall complication rate was 5.8% (18/310), some of them being severe complications such as soft tissue, bone, or brain necrosis.
A way of compensating for the limitations of external radiotherapy is the use of interstitial brachytherapy with iridium-192 wire implants Daly published the results obtained with iridium implants on 165 patients. The local control was of 96% (6/165 recurrences). Acute side effects were present in 49/165 cases (30%) at 8 weeks after treatment, and in 3% of the patients they remained until the fourth post-therapeutic month. In most cases, cosmetic and functional results were considered to be very good or good. Nevertheless, severe long-term side effects were found in 30 patients (18%), with some requiring amelioration by surgery.
HDR brachytherapy equipment made possible the use of external surface molds once again, in order to obtain the highest local control with minimal complications, in a similar way as was obtained with paraffin and wax molds with radium or radon seeds. Two kinds of applicators have been designed: one by Brock in 1992, and the other by Svoboda in 1995. In the first, the radioactive source was placed in a fixed active position at the center of the applicator 15 mm from the skin surface. In the second type of external applicator, plastic tubes were placed inside a slightly elastic silicon block up to 24 cm2. None of these applicators could be used to treat irregular or curved surfaces.
With the applicator that we describe in the present work, we tried to avoid the limitations of the preceding techniques, in a way similar to the applicator described by Syndikus to treat the palate. As it is completely after-loadable, it offers complete radioprotection. There is no personal exposure to radiation at all. As it is external, it eliminates the trauma of inserting needles or catheters as well as local anesthesia. As the external mold is designed on the patientsí masks, it is not necessary to have the patient present for a long time. Furthermore, it can be tested using many different geometries or weights of the active dwell positions until the best dose distribution is obtained in each case. With dose distribution optimization, it is possible to increase or reduce the quantity of radiation administered to any given point; for example, to increase the depth dose in a deeply infiltrated area or to protect a critical structure, for example, the eye globe. In order to increase the protection of a critical structure, lead sheets can be placed in the mold in the appropriate direction, for example, near the eye. Before beginning treatment, we can check, assess, and document the accuracy of the dose distribution calculations by doing autoradiographs of the applicator or placing TLD capsules in the patientís mask at the dose points previously introduced in the planification computer. There is no possibility of mistake due to the misplacement of the mold, or its mobility during treatment, due to its fixation with elastic bands. The patient can collaborate to put the mold in place. Treatment can be done following a fractionated and outpatient scheme, thereby avoiding the expenses of the surgery room, and the use of shielded brachytherapy rooms with restrictions of visits, making this treatment more competitive and patient-friendly.
With the use of dose distribution optimization routines and placing a sufficient number of plastic tubes at small distance between them, the surface dose is very uniform. Dose uniformity increases at 5-mm depth. The difference between the maximum and minimum dose at 5-mm depth is less than 3%, a value that is impossible to obtain with external beam radiotherapy using electrons or superficial X-rays, in the cases of non-flat surfaces, in which there are always skin areas that are closer to the beam focus. In the edges of the treated volume, the dose gradient is sharp enough to reduce the dose received in the neighboring normal tissues to negligible values. In those cases in which higher assurance of dose reduction is needed, a lead shield can be easily glued to the applicatorís surface, warranting a quality assurance of dose protection during the whole treatment. The custom-made skin molds for HDR brachytherapy fulfill all the criteria of radioprotection, patient comfort, accuracy of dosimetry, daily reproducibility, and dose distribution, making it an ideal treatment of skin carcinomas.
Radiotherapy has the theoretical advantage over surgery to allow one to treat a wider area with little alteration of normal tissues, so that the macroscopic tumor volume as well as the microscopic extension and a safety margin can be included in the high dose volume, with a high chance of success and a low probability of sequelae.
It is important to insist on the importance of accomplishing the treatment shortly after initial diagnosis. In our study, 46% of the patients were recurrences of a previous treatment, either surgery, electroresection, or Mohsí chemosurgery. These patients with recurrent tumors had a worse local control, since 2 of 3 recurrences involved recurrent tumors. The actuarial local control at 4 years was 87%, similar to the results published by Lovett, Menn , Sakkura et al. (and Griep, ranging between 73 and 89%. If we consider only the patients treated for primary tumors, the actuarial local control rate was 99%, data that compare favorably with 95% of Lovett and 94% of Fitzpatrick
Lovett reported that patients treated with electrons had worse local control than those treated with superficial X-rays. Similar results were described by Griep (90% and 93% respectively), even though they did not reach statistical significance. Data from our series show a local control similar to that obtained by Lovett with superficial X-rays.
Frequently, patients initially present with an extensive lesion that has been ignored for years or was self-treated by the patient with home remedies. In our study, 10% of the patients had lesions larger than 5 cm in diameter. These large lesions had a greater probability of local recurrence. Of the 16 previously untreated patients with lesions larger than 4 cm in diameter, 1 recurred. The 5-year actuarial control rate was 91%, a value very similar to the one reported by Lovett of 92%.
In our study there had not been cases of moderate or severe late complications. Lovett found a total of 18 severe complications in a group of 310 patients with skin carcinomas treated with radiotherapy (electrons and superficial X-ray). Mainly they were soft tissue necrosis; there were 3 cases of bone radionecrosis. The majority of soft tissue necroses developed in patients with recurrent tumors in the scalp. In 1 case the soft tissue necrosis was in the brain. The fact that in our series late complications have not been detected is probably explained by the size of dose per fraction used, since Lovett employed a dose per fraction of 300 to 400 cGy or greater, whereas we administered 180 cGy per fraction. Lovett described a smaller local control of the tumor with dose per fraction lower than 200 cGy, data that are not supported by our results, perhaps because we administered a greater total dose.