Two recent reviews make the
following comments on therapy:
Treatment of cutaneous
lymphomas
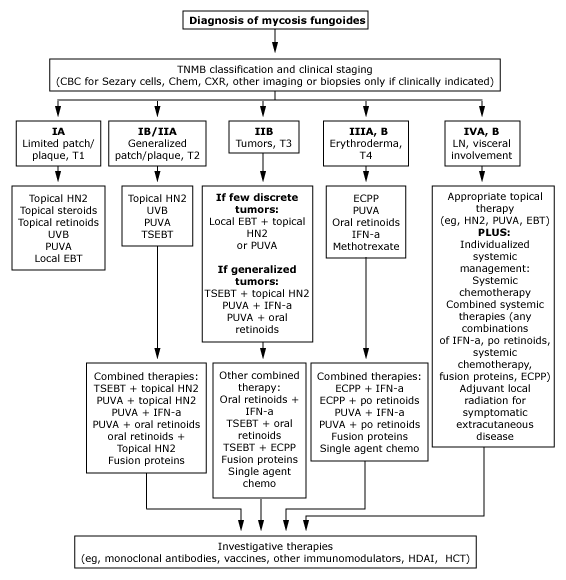
Mycosis fungoides
The most common lymphoma of the skin,
mycosis fungoides, is a cutaneous T-cell NHL. Characterized initially by indolent clinical
behavior with limited erythematous patch and plaque lesions, mycosis fungoides gradually
progresses to generalized skin involvement, tumor formation, and nodal involvement.
Cutaneous T-cell NHLs can be treated with a variety of topical and systemic therapies, but
the potential for cure is low except for very early-stage disease.
Early-stage disease Treatments
directed at the skin, such as total-skin electron-beam radiation, topical chemotherapy
with mechlorethamine or carmustine (BCNU [BiCNU]), and psoralen with ultraviolet A
activation (PUVA), are usually used as initial therapy for early-stage disease.
Extracorporeal photochemotherapy (photopheresis) is most effective in patients with
generalized erythroderma and the SÚzary syndrome.
Radiation therapy. Cutaneous
lymphomas are highly sensitive to radiation. Individual symptomatic lesions are well
palliated with small electron-beam or orthovoltage radiotherapy fields at doses of 2,000
cGy over 2 weeks. Curative radiation therapy involves total-skin electron-beam
irradiation. The six-field technique of Stanford University can treat the entire skin
surface to a dose of 3,600 cGy in 9-10 weeks. This very demanding approach requires the
patient to stand for approximately an hour per day, 4 days per week. Most responses are of
short duration.
Chemotherapy and combined-modality
therapy Single-agent and aggressive combination chemotherapy regimens can lead to
complete responses in 30%-50% of patients. However, most responses are of short duration.
A randomized trial showed no survival advantage of total-skin electron-beam radiation plus
combination chemotherapy over a conservative approach consisting of sequential topical
therapies.
Thus, state-of-the art therapy is
generally considered palliative, and most patients are candidates for clinical trials
evaluating new treatment approaches, such as interferons, retinoids, immunoconjugates, and
purine analogs. Current studies at the City of Hope National Medical Center include the
use of combined photopheresis and interferon and denileukin diftitox (DAB389IL-2
[Ontak]), an immunotoxin directed against the interleukin-2 (IL-2) receptor (see
“Immunotoxins”).
Cutaneous T-Cell Lymphoma: Pathogenesis and
Treatment
Michael Girardi, MD and Richard L. Edelson, MD
Yale University School of Medicine, New Haven, Connecticut
Oncology Vol 14, No 7, July 2000
Treatment
An understanding of the pathogenesis of CTCL helps
guide the selection of therapy.. In our working hypothesis of pathogenesis (see above),
the malignant T cells are critically dependent on stimulation by epidermal Langerhans
cells. Thus, while the presence of a T-cell clone may be demonstrable even in early-stage
patch/plaque CTCL, skin-directed therapy is highly effective. Conversely, as the T-cell
clone grows more independent of the need for this stimulation, and is more likely to cause
leukemia (as in SÚzary syndrome), systemic therapies, including immunomodulatory and
cytotoxic treatments, must be utilized.
Commonly used treatments for early-stage
(patch/plaque) CTCL include topical corticosteroids, PUVA and ultraviolet light B (UVB).
Total-skin electron-beam therapy is indicated for widespread infiltrated plaque and
tumor-stage disease. Low-dose methotrexate is often useful for resistant patch/plaque CTCL
and erythrodermic CTCL. Interferon-alfa is indicated for methotrexate failures and
recurrent tumors following total-skin electron-beam therapy.
Photopheresis may be helpful for early-stage
erythrodermic CTCL but is very costly. Retinoids may be of value for early and moderately
advanced CTCL, particularly in combination with other therapies, such as interferon-alfa
and PUVA. Systemic disease usually requires combination chemotherapy, such as that used
for non-Hodgkin’s lymphoma. However, responses are usually short lived.
Skin-Directed Therapy
For limited (< 20% body surface area)
patch/plaque CTCL, topical chemotherapeutic agents (eg, mechlorethamine [Mustargen],
carmustine) have a proven track record of efficacy.[45,46]. These agents are limited by
the development of irritant and allergic contact dermatitis.
In addition, several studies have shown the
utility of topical corticosteroids and topical retinoids in the management of early
patch/plaque CTCL. However, we recommend PUVA or UVB as a first-line therapy for most
cases of patch/plaque CTCL, since phototherapy treats even subclinical lesions. Treatments
can be tapered slowly from three times weekly to once monthly.
For patients whose disease proves resistant to
phototherapy or who have more extensive (> 50%) body surface involvement, total-skin
electron-beam radiotherapy may be warranted. Despite the fact that early CTCL forms appear
to depend on the skin environment for continued stimulation and are therefore treatable
with skin-directed therapies, close patient monitoring for disease progression is
essential.
Systemic Therapy
Cutaneous T-cell lymphoma is a malignancy of
slow-growing, mature T cells that continue to function, as evidenced by their ability to
(1) cycle between skin, blood, and lymph node compartments; (2) secrete and respond to
cytokines; and (3) interact with other cells of the immune system (eg, CD8-positive T
cells, Langerhans cells). For these reasons, CTCL responds to immunomodulatory treatments.
• Photopheresis, or extracorporeal
photochemotherapy, first demonstrated efficacy in the treatment of SÚzary syndrome in
1980.[46] The therapy consists of removing a portion of the patient’s blood via an
intravenous needle, separating out the leukocytes by centrifugation, and exposing these
cells to UVA light in the presence of psoralen. The photochemically altered cells, as well
as the untreated red blood cell and plasma portion, are reinfused through the same
intravenous needle from which they were obtained. The observation that patients can attain
a complete remission with photopheresis alone, despite the fact that approximately 10% of
the peripheral leukocytes are exposed to treatment during each treatment session
(typically given on 2 consecutive days every 4 weeks), is consistent with a tumor
vaccination mechanism.
• Bexarotene—Oral bexarotene
(Targretin) is a retinoic X receptor (RXR)–specific retinoid that has demonstrated
efficacy when used as monotherapy in CTCL regardless of stage, and was recently approved
by the Food and Drug Administration (FDA) for the treatment of CTCL. For patients who do
not respond to or cannot tolerate skin-directed therapies such as PUVA or electron-beam
therapy, oral bexarotene is a practical alternative.
While patients with erythrodermic CTCL will show
skin improvement when treated with bexarotene, it is important to note that the drug has a
limited effect on any associated leukemia. Close monitoring for hypertriglyceridemia and
hypothyroidism is necessary. The role of bexarotene in combination therapy has yet to be
determined.
• DAB389IL-2 (Ontak), a
“magic bullet” of a 389-amino acid portion of diphtheria toxin conjugated to
interleukin-2 (IL-2), was recently approved by the FDA for the treatment of CTCL. Since
CTCL is a lymphoma of mature, activated T cells, the malignant cells often express the
IL-2 receptor. Such cells readily take up the DAB389IL-2 molecules; once inside
the malignant cells, the toxin is cytoplasmically cleaved and can inhibit cell protein
synthesis. DAB389IL-2 is administered intravenously at a dose of 9 or 18
mg/kg/d for 5 days every 3 weeks for up to 8 cycles.
DAB389IL-2 demonstrated a 10% complete
response rate and a 30% partial response rate, in patients with CTCL who had received a
median of five prior therapies. Toxicities of hypersensitivity reactions and capillary
leak syndrome can be attenuated though premedication with systemic corticosteroids. |